
WATCHING OVER LIFE

WATCHING OVER LIFE
Our Mission is to develop, manufacture and deliver on-time
high-quality, easy-to-use and reliable IVD assays
for the clinical diagnosis.
Sentinel Diagnostics is an Italian company specialized in the development and the production of In Vitro Diagnostics.
Established in 1983 by two Italian independent entrepreneurs, Sentinel Diagnostics has a long and proud history as an independent IVD developer and manufacturer.
Sentinel Diagnostics is the first IVD manufacturer in Italy and it is among the biggest in Europe in its areas of activity. The aim of our daily work is to provide high quality and reliable IVD products for the clinical diagnosis.
Sentinel Diagnostics distributes its products through the major players in the diagnostic field, as well as with a network of committed, established, independent distributors.
- We are an Italian company with forty years of experience, which develops, produces and supplies its products all over the world.
- We have a Quality Management System that complies with ISO 9001: 2015, ISO 13485: 2016 and EN ISO 13485: 2016 and ISO 13485: 2016 MDSAP (Australia, Brazil, Canada, United States and Japan);
- We are a fast-growing company, which employs more than 200 highly qualified people
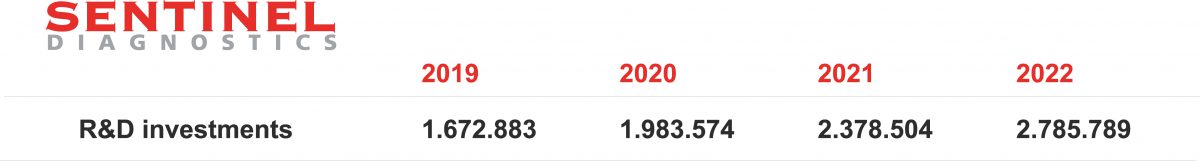
- We believe in research and continuous improvement; for this reason, every year we invest a significant part of our revenues in research and development, to always offer the best innovative solutions.

The Clinical Chemistry and Immunochemistry division is the largest and the first born in Sentinel Diagnostics.
Our specialties and latex based reagents are world renowned and appreciated, since we provide high quality assays, designed or adapted for the most widespread Clinical Chemistry analyzers.
We offer a complete product portfolio, which comprises Sentinel branded products, private labels and OEM.
Our full customization capabilities, together with the high manufacturing capacity, made us a trustful and strong partner in developing and supplying Clinical Chemistry and Immunochemistry assays.

Our Fecal Immunochemical Testing division was created around Sentinel Diagnostics’ patented universal tube for the collection of fecal samples, which has been specifically designed to be used on any Clinical Chemistry analyzer.
Its benefits include:
- easy collection: the fecal sample can be easily collected by the patient;
- sample stabilization: the sample is stabilized over time thanks to the specific extraction buffer inside the tube;
- single step analysis: the tube can be directly inserted in the rack of any Clinical Chemistry analyzer. No pre-analytical step needed.

The Molecular Diagnostics line was born in 2008 and has experienced an extensive growth over the years.
Our molecular portfolio is built on the proprietary technology STAT-NAT (Stabilized Amplification Technology – Nucleic Acid Testing), which is based on a new protective compound, able to stabilize the activity of enzymes for a very long time and to allow storage and shipment at room-temperature.
The Molecular Diagnostics line has recently expanded with new tests. Now, it includes the virology panel that contains a test for the detection of SARS-CoV-2 infection (COVID-19).

Sentinel Diagnostics’ production plant covers each stage of manufacturing: from the treatment of water, which is the basic component of all our products, up to reagent preparation, bottling, labeling and packaging.
We guarantee lot to lot consistency using standardized preparation procedures, process control and through the verification of the performance of each reagent on the most widespread analyzers, available in our validation laboratories.
We are very flexible in the realization of products according to client specification, in order to accomplish every need.
Our main objectives are:
- Understanding the client’s needs and satisfying them with timely and reliable responses;
- Continuously improving our products;
- Ensuring the adequateness of procedures and optimizing organizational processes;
- Increase the investments in research and development for more innovative diagnostics.
Sentinel Diagnostics meets the certification requirements at an international level, since we are compliant with ISO 9001:2015, ISO 13485:2016, EN ISO 13485:2016 (specific to medical devices, including IVDs), ISO 13485:2016 MDSAP (Australia, Brazil, Canada, USA and Japan), ISO 45001:2018 and ISO 14001:2015.
All these certificates have been issued by the BSI (British Standards Institution), which is one of the most recognised certification bodies in the world.
Our products and manufacturing facilities are also registered with the FDA, thus we can promote and sell our products in the US.
ISO 9001:2015
Quality Management Systems Requirements
ISO 13485:2016 & EN ISO 13485:2016
Quality Management Systems Requirements for regulatory purposes
ISO 13485:2016 MDSAP
Medical Device Single Audit Program for USA, Canada, Australia, Brazil and Japan.
ISO 45001:2018
Occupational Health & Safety Management System
ISO 14001:2015
Environmental Management System

Complying to 21 CFR 820
“Code of Federal Regulations“ FDA (U.S. Food and Drug Administration)

Moreover Sentinel is a Corporate Member of the IFCC (International Federation of Clinical Chemistry and Laboratory Medicine ) with a designated representative within the company.

Sentinel is accredited as a Validated Supplier by the IRMM (the European Commission Institute for Reference Materials and Measurements) and sponsors the research program of CIRME, the University of Milan’s Centre of Metrological Traceability in Laboratory Medicine.
![]()
We were among the first of many diagnostic firms in the European Community to observe the CE IVD regulations.
Our RA team is committed to rendering our products compliant with the regulations of many non EU countries, through the process of continual growth and adaptation to the global market.
![]()
Sentinel Diagnostics’ IT staff works to keep the company up-to-date and efficient, through the continuous technological improvements and the study and implementation of new IT projects.
IT in Sentinel Diagnostics also means easy access to technical documentation, thanks to the program Sentinel Doc, which is available both for employees and clients.
![]()
We ensure the best product handling in our warehouse through:
- Storage in controlled conditions with automatic registration of temperatures;
- The use of proper packaging material to guarantee safe transport;
- The use of freight forwarders, specialized in temperature controlled shipments;
- Temperature monitoring during transit.
![]()
We monitor the market evolution in order to catch newborn needs and develop improved diagnostic tests, able to satisfy them.
We actively participate in congresses, workshops and scientific seminars at an international level and we contribute in a concrete way to aid the diffusion of knowledge.
![]()
Our sales team employs the experience gained over thirty years, guaranteeing a complete customer satisfaction through:
- Observance of contractual terms and conditions;
- Constant supply conditions over time;
- Availability of products with long residual shelf life;
- Worldwide presence through qualified distributors at an international level.
![]()
Sentinel Diagnostics guarantees continuous support to its clients through a team of Product Specialists, who provide:
- product training;
- technical support;
- on-line support;
- up-to-date technical documentation easily accessible through Sentinel Doc.
We have adopted an Organization, Management and Control Model aimed at defining a structured system of rules that must be followed by the enterprise to pursue the social aim, in accordance with the statutory provisions in force and the Code of Ethics. The Supervisory Board has been appointed in order to supervise the compliance with the Model, in conformity with the Legislative Decree 231/01.
Our Code of Ethics identifies the general rules of behaviour that must be observed by administrators and employees, in order to guarantee compliance with the laws, regulations and the moral principles on the basis of the relationship between individuals, societies and institutions. This document is in compliance with Confindustria Dispositivi Medici Code of Ethics.
We have a privacy management system developed and optimized in accordance with Regulation (EU) 2016/679 (GDPR) on the protection of natural persons, with regard to the processing of personal data and on the free movement of such data.
Sentinel Diagnostics aims to satisfy its Stakeholders in meeting their needs punctually, with quality and reliability, according to Ethical, Quality, Health, Safety and Environmental values.















